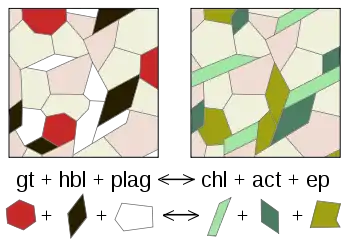
Schematic representation of a metamorphic reaction. Abbreviations of minerals: act = actinolite; chl = chlorite; ep = epidote; gt = garnet; hbl = hornblende; plag = plagioclase. Two minerals represented in the figure do not participate in the reaction, they can be quartz and K-feldspar. This reaction takes place in nature when a mafic rock goes from amphibolite facies to greenschist facies.
A metamorphic reaction is a chemical reaction that takes place during the geological process of metamorphism wherein one assemblage of minerals is transformed into a second assemblage which is stable under the new temperature/pressure conditions resulting in the final stable state of the observed metamorphic rock.[1]
Examples include the production of talc under varied metamorphic conditions:
- serpentine + carbon dioxide → talc + magnesite + water
- chlorite + quartz → kyanite + talc + water

Epidotisation in Argyll and Bute, U.K
Polymorphic Transformations
Exsolution Reactions
Devolatilization Reactions
Continuous Reactions
Ion Exchange Reactions
Oxidation/Reduction Reactions
Reactions Involving Dissolved Species
Chemographics
Petrogenetic Grids
Schreinemakers Method
Reaction Mechanisms
See also
Notes
- ↑ "Types of Metamorphic Reactions". Tulane University. Retrieved 2007-06-22.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.